Article: On thermodynamic and microscopic reversibility
Gavin E. Crooks, J. Stat. Mech. (2011) P07008
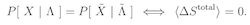 Abstract
The word ‘reversible’ has two (apparently) distinct applications in statistical thermodynamics. A thermodynamically reversible process indicates an experimental protocol for which the entropy change is zero, whereas the principle of microscopic reversibility asserts that the probability of any trajectory of a system through phase space equals that of the time reversed trajectory. However, these two terms are actually synonymous: a thermodynamically reversible process is microscopically reversible, and vice versa.
Abstract
The word ‘reversible’ has two (apparently) distinct applications in statistical thermodynamics. A thermodynamically reversible process indicates an experimental protocol for which the entropy change is zero, whereas the principle of microscopic reversibility asserts that the probability of any trajectory of a system through phase space equals that of the time reversed trajectory. However, these two terms are actually synonymous: a thermodynamically reversible process is microscopically reversible, and vice versa.