Article: Protein–ligand binding free-energy calculations with ARROW – A purely first-principles parameterized polarizable force field
Grzegorz Nawrocki, Igor Leontyev, Serzhan Sakipov, Mikhail Darkhovskiy, Igor Kurnikov, Leonid Pereyaslavets, Ganesh Kamath, Ekaterina Voronina, Oleg Butin, Alexey Illarionov, Michael Olevanov, Alexander Kostikov, Ilya Ivahnenko, Dhilon S. Patel, Subramanian K. R. S. Sankaranarayanan, Maria G. Kurnikova, Christopher Lock, Gavin E. Crooks, Michael Levitt, Roger D. Kornberg, and Boris Fain, J. Chem. Theory Comput (2022)
[ Full text ]
Abstract
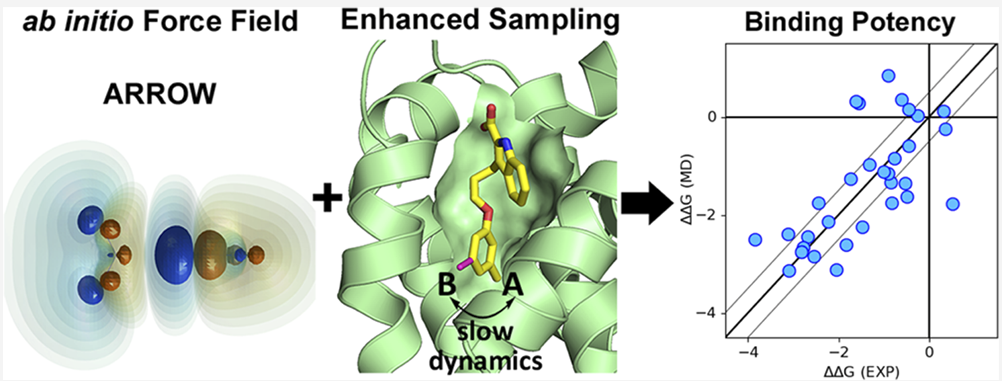
Protein−ligand binding free-energy calculations using molecular dynamics (MD) simulations have emerged as a powerful tool for in silico drug design. Here, we present results obtained with the ARROW force field a multipolar polarizable and physics-based model with all parameters fitted entirely to high-level ab initio quantum mechanical (QM) calculations. ARROW has already proven its ability to determine solvation free energy of arbitrary neutral compounds with unprecedented accuracy. The ARROW FF parameterization is now extended to include coverage of all amino acids including charged groups, allowing molecular simulations of a series of protein− ligand systems and prediction of their relative binding free energies. We ensure adequate sampling by applying a novel technique that is based on coupling the Hamiltonian Replica exchange (HREX) with a conformation reservoir generated via potential softening and nonequilibrium MD. ARROW provides predictions with near chemical accuracy (mean absolute error of ∼0.5 kcal/mol) for two of the three protein systems studied here (MCL1 and Thrombin). The third protein system (CDK2) reveals the difficulty in accurately describing dimer interaction energies involving polar and charged species. Overall, for all of the three protein systems studied here, ARROW FF predicts relative binding free energies of ligands with a similar accuracy level as leading nonpolarizable force fields.